Articles
Daniel Gastfriend: Manufacturing medical countermeasures against catastrophic biothreats
Daniel Gastfriend: Manufacturing medical countermeasures against catastrophic biothreats
The United States is underprepared for a future pandemic. A recent simulation conducted by the Johns Hopkins Center for Health Security found that an engineered pathogen could overwhelm U.S. and global response capacity, killing more than 150 million people within 20 months. One important reason: The U.S. lacks the capacity to manufacture medical countermeasures (MCMs) fast enough to contain a global outbreak.
In this academic session, Daniel Gastfriend, a recent visiting scholar at the Johns Hopkins Center for Health Security, discusses three categories of emerging technologies that could improve our MCM production capabilities: flexible manufacturing, platform technologies, and localized distributed manufacturing.
Below is a transcript of Daniel’s talk, which we’ve lightly edited for clarity. You may also watch it on YouTube or discuss it on the EA Forum.
The Talk
First, a few quick notes: My background and expertise are in economics and business. I'm not a biologist or a scientist, so I'm going to focus on the policy and business aspects of preparing for pandemics rather than the technical aspects. Also, I've conducted the research I’m presenting in partnership with the Johns Hopkins Center for Health Security, but the views I will express are my own.
Let's begin. [Imagine] the year is 2025. You wake up at 6:00 AM in the morning to your phone ringing. You hear the voice of the chief of staff of the White House. She says, “You're on the line with the president of the United States of America.”
“Good morning,” says the president. “As you know, we are in the midst of a terrifying pandemic outbreak of the Clade X virus that is threatening to destroy the global system. We urgently need you to report to the White House to help coordinate the U.S. response.”
You say, “Okay.” You throw on your pants, jump in your car, and drive to the White House, where you're greeted by the chief of staff.
She says, “Let's get you up to speed.”
[Daniel plays a fictional video with voiceover from actors portraying a newscaster and government officials:]
_“We are continuing our coverage of a new and deadly infectious disease, [the Clade X virus, which was intentionally created and released]. The virus has some genetic elements of the NEPA virus.” _
_“The care of these patients requires extraordinary effort.” _
_“We cannot and will not voluntarily take patients from other hospitals.” _
_“The impact of not doing something has lots of consequences.” _
_“We can't retreat from the rest of the world.” _
_“Continuity of government here cannot be overestimated.” _
_“Federal quarantine of this scale is unprecedented.” _
_“The question everywhere: When will there be a vaccine?” _
_“We must engage the private sector.” _
_“[People] know that we don't have a vaccine yet. They want vaccines to be prioritized.” _
_“I would not want to pull those people back.” _
_“With the need on the ground, can we assure protection of our first responders?” _
“This is a nonstarter. I'm going to need these people [first responders] — I’ve got this feeling — in a whole lot of other places.”
_“More than 40 countries are reporting outbreaks and many more are suspected of having cases.” _
_“Leadership requires doing things that are oftentimes unpopular.” _
_“This issue has the amazing capacity to be number 11 on anybody's list of the 10 most important items.” _
_“We have been unbelievably weakened by this crisis.” _
“What the world will look like when it's done is still very uncertain.”
[Video ends.]
This was a tabletop exercise conducted by the Johns Hopkins Center for Health Security with current and former senior members of the U.S. government.

They ran epidemiological modeling and predicted that within a year or so of the release of this type of virus, there would be 150 million deaths worldwide and a 50% drop in global GDP.
If these numbers appear historically unprecedented, they're not.

We often forget that the biggest killers in the 20th century were infectious disease outbreaks. The 1918 Spanish flu, in a single year, killed more people than World War II. Could a pandemic outbreak be even worse than this? Could it permanently harm and alter the trajectory of human civilization — or pose a truly existential threat to human society?
There's legitimate and ongoing debate about how seriously we should take that risk. I will offer two historical anecdotes to inform your thinking.
In 2012, scientists were working on the H5N1 avian flu virus. It's a variant of the flu with extremely high case-fatality rates, but it's not transmissible between mammals through respiratory pathways. The scientists [tweaked the virus such that it became] transmissible by air. Subsequent modeling suggests that the accidental release of this virus from a lab could kill upwards of one billion people.
In 1993, the omnicidal cult Aum Shinrikyo, in their efforts to exterminate humanity, attempted a wide-area anthrax attack in Tokyo.

Luckily, the group wasn’t capable enough to successfully execute the attack. But the combination of the increasing capabilities of synthetic biology to create devastating pathogens and the presence of individuals and groups who would use them against society is something that should concern us all.

[Let’s return to the tabletop exercise.] You're at the White House in your first meeting with the president of the United States. The president says, “Tell me, what can we be doing to better prepare ourselves? And what should we have been doing all along?”
You quickly run through a list. We could:
- Invest in basic research to better understand the biological pathways and mechanisms of disease.
- Build our arsenal of medical countermeasures against potentially catastrophic pathogens.
- Improve governance, planning and coordination. There are key gaps within both federal government and international entities [that inhibit our ability] to manage an effective response.
- Improve our allocation of resources and target them toward the most severe and significant threats, especially synthetic biology.
- Improve our regulation of synthetic biology to prevent the wrong tools and pathogens from falling into the wrong hands.
- Strengthen international diplomacy around biological security; [for example, through] the Biological Weapons Convention.
The president says, “That's a great list. But it's too late for most of this now, so let's focus on developing and deploying a medical countermeasure.”

[For the purposes of this fictional exercise], let's assume that the government has already developed a vaccine. The question now is: How quickly can we manufacture this vaccine and deploy it globally?

The president says, “The international economy is collapsing. Millions of people are dying by the week. I want you to manufacture enough vaccine doses to cover the majority of the global population in the next three months. Can you do it?”
You look at the president and say, “You're not going to like the answer to that question.”

[Although I’m focusing this talk on] manufacturing, development of countermeasures is also crucial because it can take 10 to 15 years to produce a vaccine. But even under the optimistic scenario in which we have a vaccine, manufacturing is going to be way too slow to respond.
The 2009 flu pandemic [led to] one of the fastest production and scale-ups of a vaccine in history. Yet it still took nine months after the first case to [achieve large-scale vaccine distribution].
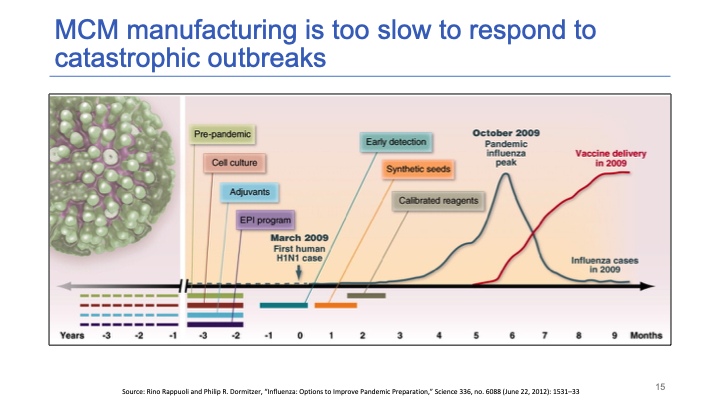
The blue line represents the course of the viral outbreak and the red line is a scale-up of vaccine delivery. There was a three month delay between the peak of the outbreak and vaccine delivery reaching full [capacity]. And that was the best that we have ever managed.
It turns out that the flu vaccine is the vaccine for which there is the most global capacity [in place] to respond to a global pandemic. Our capabilities have improved since 2009.

This slide summarizes the question of whether we have the capacity to cover the global population — or at least a majority of the population — in 12 months. What's the current excess capacity to produce the vaccine, and how quickly can we scale up?
For the flu vaccine, we can't quite cover the global population in 12 months, but under very optimistic assumptions we can get within striking distance. Unfortunately, no other vaccine comes anywhere close to hitting those numbers, and it can take three to five years to build a single new factory for vaccine production. Plus, it can take between six to 12 months to repurpose a factory’s existing capacity from producing one vaccine to another.
The figures are similar for most other categories of countermeasures — the one exception being broad-spectrum antibiotics. If the bacteria happens to be susceptible to penicillin, then we might be in decent shape. If not, we're really screwed.
[Let’s] rewind five years [from the fictional scenario] to 2019. You get a very different call from the president of the United States, who says, “I know that we should be concerned about pandemic outbreaks. I know we should be concerned about synthetic biology. What can we do today to build out our manufacturing capabilities so that if a crisis occurs, we'll be better prepared?”

First, it'll be useful to identify the main barriers preventing us from having improved surge-production capabilities.

Some barriers are technical. Most processes to produce medical countermeasures rely on expensive fixed equipment, and they are very specialized processes that require specific knowhow. It can take months for a team to get used to the specific manufacturing process for any given production line, such that it's quite difficult for a factory producing one product to switch to another product. It is possible, but tends to be challenging.

Other barriers are economic. It would be extremely socially valuable to invest in surge-production capacity to help insure against an extreme-risk scenario. But it's difficult for private companies to internalize those benefits as a return on their investment. This is because they would need to recoup quite a substantial financial investment in a crisis that might only happen once every 50 years. And the prices that they would have to charge to justify their investment, from an economic perspective, would likely be viewed politically as exorbitant and unacceptable. It’s not a play that any private investor is going to be excited to make.

Finally, there are regulatory barriers. There are extremely stringent regulations of the manufacturing of medical countermeasures; these regulations are called “good manufacturing practices.” They are really important for protecting users of vaccines, antibiotics, and other health products during peacetime. They’re why vaccines have such high safety rates. But they can slow down scale-up in an emergency, especially because there are [regulatory] differences internationally.

Next, I'm going to present three potential policy strategies that we think could help alleviate these barriers. I'll run through them quickly, and then dive more deeply into the first one.

The first strategy is to promote platform technologies. These are a category of manufacturing technologies that could be much more flexible and rapidly scalable than conventional manufacturing. They would have transformative impacts on technical and economic barriers, but it would likely take five to 15 years to get these products on the market. It would require an investment of at least several billion dollars, if not much more than that.
Second, there's a slew of ideas that could bolster the capabilities of the federal government to respond to an emergency and invest in countermeasures.
The third strategy involves improving planning between the public and the private sectors to make sure we have strong emergency response plans.
I want to return to platform technologies because I think they are our best bet. It's useful to contrast them to conventional manufacturing. In conventional vaccine manufacturing, you typically attenuate the target pathogen against which you want to stimulate immunity. You're going to grow that pathogen — for example, a virus — in a large bioreactor or vat of cells. You extract and filter the attenuated virus, and administer it to the patient whose immune system then generates immunity against that virus. This turns out to be quite a slow and inflexible manufacturing process.

By contrast, platform technologies use a specific delivery mechanism: a platform on which you can deliver many different types of products.
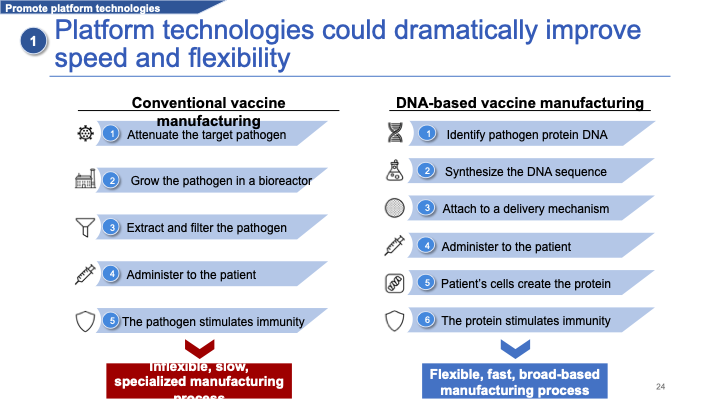
I'll use the example of DNA-based vaccine manufacturing. In a DNA-based vaccine, you identify a specific protein on the target pathogen and antigen, which can stimulate immunity. You identify the gene sequence of this protein and synthesize that gene sequence in DNA. You attach it to some delivery mechanism that can bring it into the cells. You administer it, and the patient's cells then create that protein using their own molecular machinery. It's that protein that then stimulates the immune response.
This is much faster and more flexible because it allows manufacturers to type in a new and different DNA sequence, and quickly switch from producing one product to another using the same delivery mechanism. You can switch over in weeks or maybe even days.
The problem is that we're still five to 15 years away from these products maturing. There are a few barriers that public policy could alleviate.
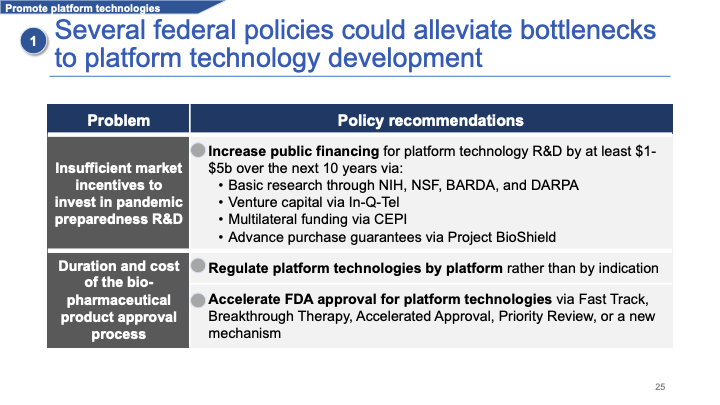
The first is insufficient private-sector investment for some of the reasons that I mentioned earlier. I think there should be massive public financing, on the order of several billion dollars over the next decade — if not much more than that — to accelerate research into these kinds of vaccines.
We could also accelerate regulatory processes for these platforms by regulating them based on delivery mechanism rather than disease type, and by using a variety of tools that the FDA has [at its disposal] to accelerate product approval.
Finally, I'll quickly talk through some ideas to strengthen the capabilities of federal government to invest and respond in this space. The federal government already partially invests in facilities that are designed for surge manufacturing. But it hasn’t yet fully delivered on its promise.

We could help alleviate that by establishing multi-year appropriations for the government to invest in medical countermeasures over the long term.
We could make [it easier for] the Department of Health and Human Services to contract with private-sector agencies and organizations, and we could allocate emergency-response funding that the federal government could draw upon immediately [if need be].

The final recommendation is to create government-industry planning forums to better coordinate emergency regulatory planning between private-sector companies and the federal government.

This last slide is just a list of ideas for anyone interested in getting involved in biosecurity.
Thank you.
Moderator: Our discussant for this section will be Greg Lewis. Greg is a scholar at the Future of Humanity Institute where he investigates long-run impacts and potential catastrophic risk from advancing biotechnology. He is a doctorate in philosophy student in Michael Bonsell's mathematical ecology group. Previously, he was an academic clinical fellow in public health medicine, where he won the O'Brien Prize. He holds a master’s in public health and a medical degree, both from Cambridge University. Before studying medicine, he represented Great Britain in the International Biology Olympiad.
Greg Lewis [Discussant]: Thank you very much — and sorry for making you have to quote my own puffery back at me.
Thank you so much, Daniel, for the excellent talk. I have two main questions, which might serve as prompts for discussion. It seems to me that the technology is currently not yet there to have sufficient capacity, no matter what regulatory or economic barriers are in place. To reserve sufficient plant capacity is going to require several orders of magnitude more of an existing aggregate medical production in the world, which seems impossible even if you spend a lot of U.S. GDP on it.
So if you were targeting policymakers, [the message would be] to simply invest more in platform technologies. The other things are nice to have at this stage, but seem to not have as much relevance. But maybe I'm over-simplifying.
Daniel: Yeah, I would broadly agree with that assessment. But I think the story is a little more nuanced than you just presented. I think there is actually another set of technologies that can be helpful in the interim. I'll just quickly point them out here.

This is quite a complicated slide, but it summarizes different technology categories based on how flexible they are and how efficient they can be at scale. [The categories at] the top left are efficient at scale but extremely inflexible, which is where we are right now.
There's another category of technologies called “flexible manufacturing technologies” that could incrementally improve flexibility. These are things like using disposable bags in your bioreactors, so that if you're producing one product and want to switch to another, you can just throw out that plastic bag and use the same bioreactor without spending three months sterilizing every single piece of your equipment. I think that for a range of plausibly bad pathogens that we might face, this could improve capabilities, so it is worth [pursuing] some interim policies to try to achieve those benefits.
Greg: What sort of surge capacity do these things currently have? What order-of-magnitude increase might you get from these intermediate technologies?
Daniel: The industry is already moving in this direction, and implementing these technologies could reduce turnover times from a factory when switching from one product to another from, say, 12 months to maybe three to six months. I think that’s a meaningful improvement. But if everyone's going to die in three months, it's not going to solve that problem.
Greg: Alas, no. I essentially agree. But tt seems like reducing the vulnerability surface even incrementally would be valuable, even if we don't yet have a single silver bullet.
One further question: The cost for some of the [strategies] you mentioned would, you said, be on average from $0.5 to $5 billion, which is on the order of the United States’ entire spending on biosecurity. So the archetypal EA question is: How would you prioritize this among the entire portfolio of biosecurity work?
Daniel: Yeah, it's a great question.
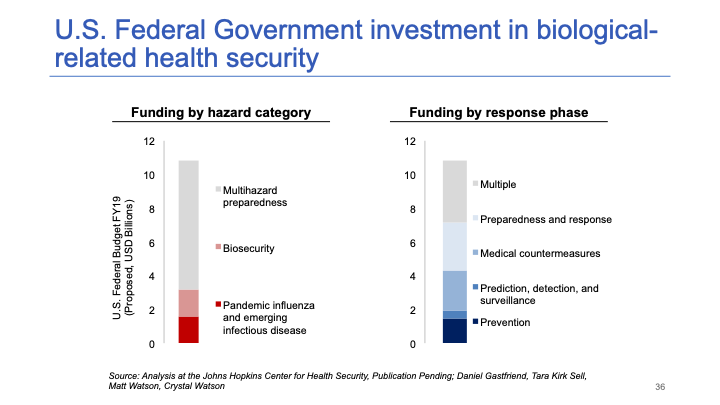
This slide summarizes U.S. investment in biosecurity, pandemic preparedness, and other types of hazards (chemical, radiological, nuclear, etc.). It's a few billion for biosecurity each year and maybe $10 billion for multi-hazard preparedness.
I would put investment in platform technologies near the top. There may be even more crucial investments we could be making in accelerating development timelines for new countermeasures — things like broad-spectrum antivirals might be even more important. But I do think investment in platform technologies would be better than some of the investments the government is currently making, and it’s certainly worth increasing this budget by $1 billion a year. A 10% increase each year would be very worthwhile.
Greg: In terms of prioritizing between the options you yourself have selected, I think you mentioned that maybe the federal government could fund something like [the Project BioShield Act] to stockpile vaccines. That feels to me to be of very limited value, because you can only stockpile a set number of vaccines, and it might cost $4 billion. I’d rather spend $4 billion on [the other ideas you mentioned]. I'm wondering how you look at it.
Daniel: Yeah. So, so my understanding of [Project BioShield] is it funds stockpiles, but it also funds the development of countermeasures and manufacturing capabilities. I could be wrong about that.
I think stockpiles are a “nice to have” but not as crucial as developing new countermeasures.
Greg: I guess we're in furious agreement. Let’s hand this over to audience questions.
Moderator: One audience member asks: Is there any possibility that some of these platform technologies that you described could also pose a dual-use concern [i.e., be misapplied and inadvertently create a threat in another area]?
Daniel: That's a really good question. Yeah, I think that advancements in delivering DNA and RNA to cells and getting that protein expressed by the cells probably could have some dual-use concerns. Based on what I've heard from scientists, I think it would be worth the risk because there are many other ways you could develop completely catastrophic pandemics. But [perhaps Greg could weigh in, since he is a scientist].
Greg: Yeah. To a first approximation, all of biotechnology is dual-use. I agree there’s a risk of misuse. That being said, you want to differentiate between [misuses]. I'm keen to [risk what Daniel has discussed] over people synthesizing pathogens from scratch as they've done in the past.
Moderator: One audience member asks: How do the research and development capabilities of other countries, such as China, compare to those of the United States? Could we potentially team up with other countries to accelerate the development of platform technologies or flexible manufacturing methods?
Daniel: It's a great question. I definitely think there should be international cooperation. My understanding is that the U.S. and Europe are much more advanced in terms of biopharmaceutical manufacturing, and China has more capacity in terms of the synthetic manufacturing of small molecules. So my suspicion is that a lot of this research would be done in the U.S. and Europe. But I'm not an expert in that area, so I could be wrong about that.
Moderator: That's all the time we have for questions. Let's thank Daniel and Greg.